Today, Clinical Research is a term that everyone has heard about. It is referred to whenever we talk of a new drug or device introduction at the market place. Till very recently, even in the later part of the 20th century, Clinical Research was largely unknown to a common man; it was referred to only by scientists and a few others in the industry.
It is interesting to know that the history of clinical trials, however, dates back to the era of 500 BC. The world’s first clinical trial is documented in the ‘Book of Daniele’ in The Bible.
From the first documented clinical trial in The Bible to the first randomized controlled of trial of streptomycin in 1946, the history of clinical trial involves different type of challenges viz. scientific, ethical and regulatory. The streptomycin clinical trial is till date recognized as a revolutionary one.
Clinical research is medical research that establishes the safety and efficacy of medicines, medical devices, diagnostic products, and treatment regimens meant for human use.
It involves the study of both new drugs and old ones to prevent, treat, and diagnose diseases, as well as to find ways to improve patient lives. In a nutshell, it helps us understand diseases better, and consequently engineer better treatment strategies, drugs, and medical devices.
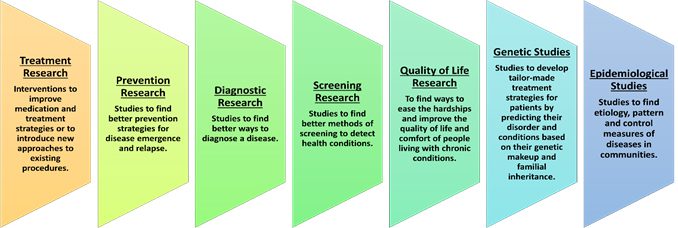
Clinical research can be classified in many ways. Based on the area of interest and what the researchers are studying, the types of clinical research are:
Clinical Research is one of steps in the broad framework of drug discovery to marketing of a new product. While we will restrict the discussion to Clinical Research, it would help to understand the steps involved which are as given below:

Before looking at what clinical trials are composed of, let’s look at two other important terms: nonclinical trials and preclinical trials. Both these also come under studies, or studies which make a drug ready for being tested in clinical trials.
Nonclinical and preclinical studies do not use human test subjects. Preclinical trials may use animal subjects for testing. At this stage, the methodology of formulation, toxicology data, stability, disintegration, and dissolution studies, pharmacokinetic and pharmacodynamic data of the drug are validated. Its interaction with living systems (tissues, cell cultures or animal models) is studied in laboratories. Thus, these studies, help in checking for any possible toxicity of the drug / device before it is tried on human subjects.
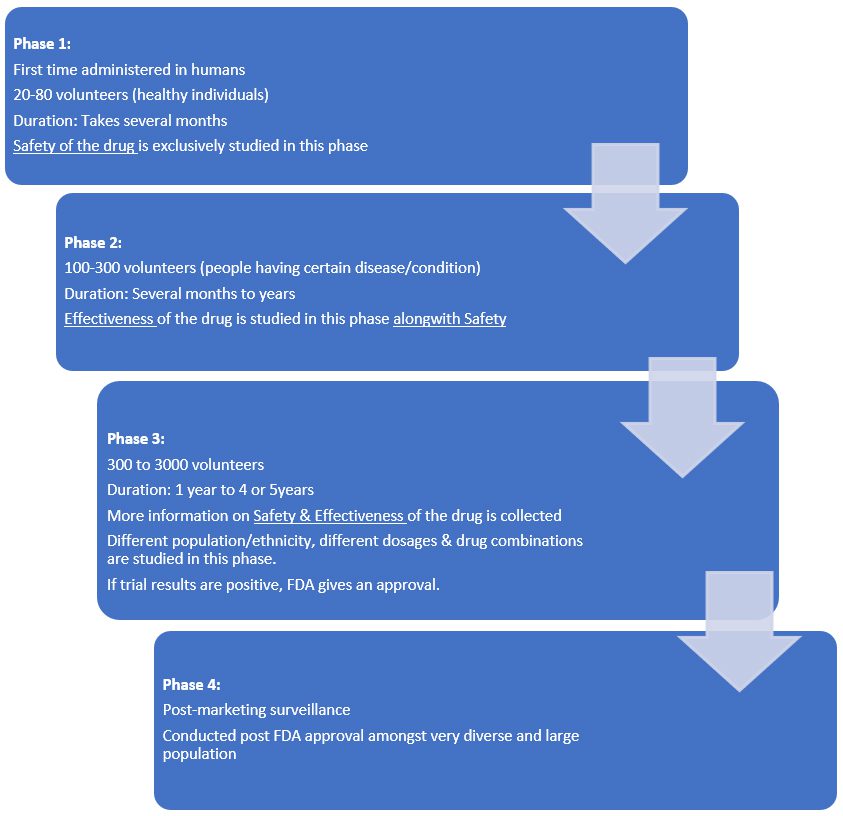
If the preclinical research results are favorable /encouraging, the approval is granted for the study to be conducted on humans in a clinical trial to test the safety, efficacy, the right dosing as well as assess the side effects.
They are primarily of two types – non-interventional & interventional studies.
(A) Observational or non-interventional studiesIn observational or non-interventional studies, no experiments are conducted among people; there is no specific or potential treatment for the volunteers. They are just observed on their current treatment plan, lifestyle, their habits and existing medications are tracked, and their health is assessed over time. The safety and potency of a drug or the functionality of a medical device in day-to-day lives of patients is generally assessed.
(B) Clinical trials or interventional studies“Interventional" means a type of clinical study in which participants are assigned to groups that receive one or more interventions/treatments so that researchers can evaluate the effects of the interventions on biomedical or health-related outcomes. The assignments are determined by the study protocol. Participants may receive diagnostic, therapeutic, or other types of interventions.
Another unique type of interventional studies are the Academic Trials, which are conducted purely for academic or research purposes for a drug that has already been approved by a regulatory body. It is usually done when a new intervention regarding its formulation, route of administration or indication needs to be researched.
Patient groups/Volunteers are identified and prepared for the study. Duration of study, number of people participating, control group selection (if required), route and dose of administration, method of collection and analysis of data collected are all determined. In this manner, groundwork for a clinical trial is laid down.
Once these initial tests are done, the drug can be deemed as an investigational new drug (IND) and can be introduced to the next stage. An approval of the IND must be obtained from the regulatory authorities to begin clinical trials.
Interventional studies, or clinical trials, undergo a rigorous process to obtain approval.
In India, the Central Drugs Standard Control Organization (CDSCO), headed by the Drug Controller General of India (DCGI), is responsible for approval, supervision, and inspection of clinical trials. In addition, responsibility of CDSCO also covers inspection of trial sites, inspection of sponsors of clinical research and manufacturing facilities in the country. In Indian regulations, the DCGI is also referred to as the Central Licensing Authority (CLA).
The DCGI also approves the imports of drugs required for use during clinical trials.
In clinical trial terminology, the individual or company which undertakes a clinical trial is known as the Sponsor. The sponsor must first apply for clinical trials. This application must contain the following data about the IND:
- Basic drug profile
- Toxicology, pharmacodynamic, pharmacokinetic profile of drug
- Preclinical study reports (studies done on an animal model, tissue, or cell line)
- Methodology of manufacture
- A plan of how the trial will be conducted
- Information about the Sponsor
A similar application can be made for new and imported drugs.
This application must be reviewed and approved both by the DCGI and an Ethics Committee (EC) registered under the DCGI.
After approval, the Sponsor must register for a clinical trial in the Clinical Trials Registry- India (CTRI). This must be done before any participant or volunteer is enrolled in the study.

Thus, the prerequisites of conducting a clinical trial in India include -
- Permission from the Drugs Controller General, India (DCGI).
- Approval from respective Ethics Committee where the study is planned.
- Mandatory registration on the ICMR maintained website www.ctri.in.
The volunteers are selected for the clinical trial based on various factors as mentioned in the protocol of the clinical trial. Various demographic factors, health conditions and medical records are studied before the inclusion of these volunteers for a study. On qualification, it is imperative for the Clinical Trial Investigator team to make full disclosure about the trial and take an informed consent from the volunteer regarding the participation.
Thus, any clinical trial process goes through an elaborate procedure before it actually begins.
CROs are companies which are hired by Sponsors (pharma companies, medical device manufacturers, biotech companies) to undertake the clinical research activities on their behalf. Thus, the work of clinical trials is outsourced by these sponsors to CROs so that the complete supervision and responsibility of the trial can be absorbed by the CROs.
The question therefore arises as to why the companies outsource it to CROs? The reasons can be many –
- they are specialists and therefore have complete knowledge of the processes and regulatory sanctions involved in the smooth completion of the trial
- they take charge of the application process, getting approvals, licences and certificates needed for the trials as well as recruiting of trial investigators and volunteers
- they ensure that the trial quality meets the national and international needs
- they reduce the trial work load of the sponsors who can therefore concentrate on their own business
- they also sometimes provide with innovative and technological solutions so that the overall trial cost is reduced
CROs therefore emerge as an important stakeholder whenever we talk about clinical trials across the globe. Most CROs are global companies with offices across various regions of the world so that the geographical needs for the trial are well known and adhered to.
Conducting Clinical research involves a multidisciplinary team, in which pharmacists form an integral part. Every stage of the clinical research process has a need for someone with the domain knowledge and hence pharmacists emerge as a natural choice.
Pharmacists are a part of the regulatory body involved in:
- review and approval of drug,
- research of the drug,
- design and managing clinical trials,
- supervising dosage, administration, and drug profiles.
- taking care of and counselling the patients and volunteers involved in the trials.
- advising volunteers on how to take the specified medication and ensure that it is being taken in the right way and the right dose. This in turn is useful to produce uniform results and facilitate easier study.
Additionally, the responsibility of checking the IND also lies with the pharmacist. The manufacturing, packaging, labelling, transport, storage, and quality control of the drug are supervised by the pharmacist. In case of anomalies, the pharmacist is expected to report the same to the sponsor.
Besides pharma companies engaging in clinical research activities, CROs also offer a vast scope for one to build a career in this function. The spectrum of work also extends to medical writing and pharmacovigilance activities, which are many times linked to the clinical research team.
Whether one is a graduate, post-graduate or a doctorate, this function opens an array of opportunities to a young pharmacist. Today, many certificate courses in this area can also be done by a graduate to upskill and get ready for a career in this function.
The global clinical trials market which was about 38.7 billion USD in 2021 is expected to grow to 52 billion USD by end of 2026 at a CAGR of 6.1%. The Indian clinical research market is estimated to touch 3.i5 billion USD by 2025 with a CAGR of 8.7% from 2021. This phenomenal growth in this industry is primarily due to the growing expenditure of global majors on R & D, increase in discovery of newer drugs, increased awareness on the need for treatment of chronic conditions. The covid 19 pandemic has only propelled the public and government attention on healthcare and the need for right drugs. And hence the growth in the clinical research expenditure.
We therefore foresee a great opportunity for people with domain knowledge to make a career in clinical research. Whether it is a pharma organization or a CRO, whether it is a pure clinical research role or as a medical writer or in a regulatory function the sky is the limit for someone entering this role - an absolutely evergreen career path for pharmacists!
- https://www.ncbi.nlm.nih.gov
- https://www.fda.gov
- https://www.nia.nih.gov
- https://rarediseases.info.nih.gov
- https://clinregs.niaid.nih.gov/country/india
- https://www.marketsandmarkets.com/Market-Reports/clinical-trials-market
- https://www.fortuneindia.com/enterprise/india-now-prime-destination-for-big-pharmas-global-clinical-trials

