“Pharmaceutical products are indispensable to humanity” - they are instrumental in the treatment, diagnosis, and cure of diseases and ailments, which would have otherwise terrified the human race. Thanks to these drugs and medicines we are capable of taking even the most ravaging of diseases head-on.
Accompanying the uses and benefits are also the undesirable effects; needless to mention one has to carefully weigh the benefits vs the risks and then decide on its use in mankind. The regulatory authorities across the world are constantly working towards ensuring that the patients receive the safest therapy at all times.
Drugs may sometimes show effects that are unexpected. In some cases, these might be damaging or even fatal for the patient population. Such undesirable effects are known as ‘adverse drug reactions’ or ADRs. Evidently, it becomes a top priority for the industry to detect such effects, and take corrective measures as early as possible.
Even after years of research and testing, a drug may exhibit an undesirable effect, which may not have been detected during the clinical trials. These effects may appear only when the drug is used by a large and diversified population and over long periods of time.
Monitoring of these ADRs after the drug/product has been commercialised is referred to as Pharmacovigilance (PV).
It is defined by the WHO as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other medicine/vaccine-related problem.”
What is Pharmacovigilance?
Pharmacovigilance is the discipline that backs us and aids us in the detection of ADRs and side effects. It contributes to the assessment of medicines- risks, effectiveness, benefit, and harm, and encourages their safe and rational use. It is designed to provide crucial data on how drugs work in medical practice, from the short-term to the long-term. This data greatly helps the researchers in drug development and marketing; thus, proving to be a boon and hence an invaluable safety net to the industry.
The need for Pharmacovigilance was first triggered by the thalidomide tragedy of 1961, where thalidomide was used to treat nausea in pregnancy, resulting in serious teratogenic events among infants exposed in utero; following this, the need for a global system for patient care and safety was realised.
How does the PV system work?
The PV team, the world over, works in collaboration with the Regulatory, Clinical trials, and Marketing functions of an organisation,
Broadly, the process of pharmacovigilance includes 4 stages: Detection, Assessment, Understanding, and Prevention of ADRs.
I) Detection involves a collection of Individual Case Safety Reports (ICSRs).
It includes:
- Pre-marketing surveillance, which comprises preclinical screening and the clinical trials
- Post-marketing surveillance, which comprises passive and active surveillance.
- Passive surveillance includes the collection of data from unsolicited sources like patients, consumers, healthcare professionals, the internet, reports, etc.
- Active surveillance includes targeted monitoring for certain events or drugs and is a pre-planned process involving the collection of data from solicited sources or established channels like registries. Clinical trials are included here. It is also known as toxicity monitoring
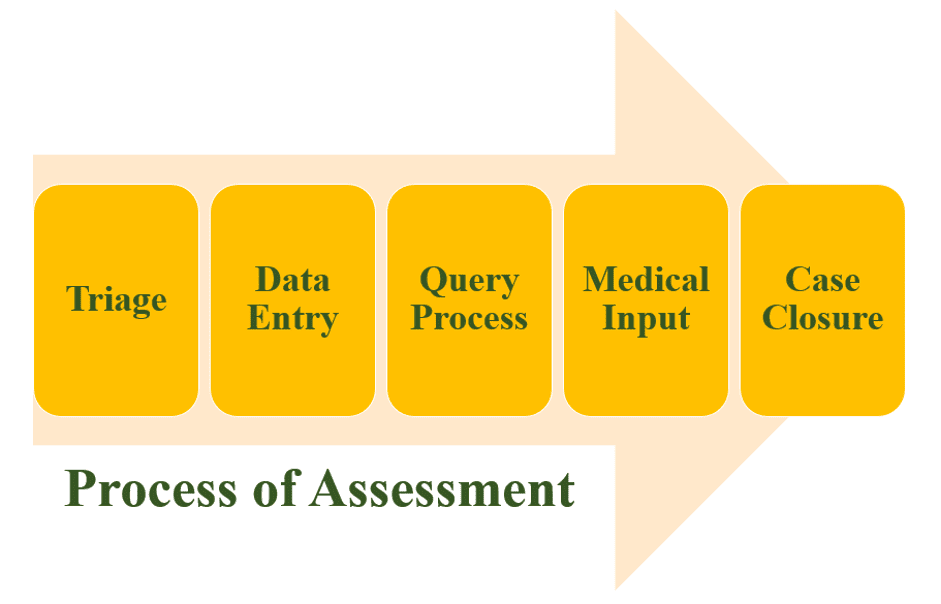
II) Assessment is a long process involving:
- triage (validating the received information)
- making a record of it in the database if it is valid
- resolving any discrepancies in the data with help from the sources.
- this data is then reviewed by a medical professional for their comments on it, following which the case is closed
- the report is then submitted to the regulatory authorities.
III) Understanding the case and the drug in question. Its safety profile is studied and updated in accordance with new data. The recorded data is stored and maintained for future perusal.
The assessment and understanding of the data are done by the ‘case processor’, who is an experienced professional capable of handling the data and performing the required tasks.
IV) Utilising the collected data to minimise ADRs. This is done by making changes in the product- the labelling, instructions, and legal aspects and by monitoring risk minimisation activities. Any amendments in previously dispensed information are made to ensure that no such instances occur again.
Pharmacovigilance in India
India has emerged as a global hub of clinical trials over the recent past and hence pharmacovigilance system in India is of prime importance for players in the health care and pharmaceutical business.
In the year 2010, the Government of India launched the nationwide Pharmacovigilance Programme of India (PvPI) with the objective of building confidence and trust across the population with respect to medicine's safety. This initiative, though a bit delayed in the country as compared to the western world, has seen significant acceptance in the past few years with rapid progress in reporting of ADRs by healthcare professionals.
The need for PvPI stemmed from the considerable social and economic consequences associated with adverse drug reactions. The positive benefit/cost ratio of implementing appropriate risk management along with the need to engage healthcare professionals and the public at large, resulted in the creation of PvPI by GOI to build synergies for monitoring adverse drug reactions in the country.
The Indian Pharmacopoeia Commission functions as the National Coordination Center (NCC) for the Pharmacovigilance Programme of India (PvPI). The objective of PvPI is – “To be the leading national authority protecting national health from adverse effects of medicine". Currently, there are 22 ADR Monitoring centres in India set up under the PvPI.
PvPI, in collaboration with WHO, has put together the PvPI toolkit. It is equipped with pharmacovigilance tools in line with the current best practices as well as the aforementioned ADR Reporting Form. PvPI works continuously to optimise the safety of medicines and prevention of ADRs due to new and existing drug treatments.
Challenges faced in Pharmacovigilance
Pharmacovigilance is the way forward if we want to ensure the safety of our population and efficacy and rational use of drugs and medicines. However, as any other system, Pharmacovigilance also faces a few challenges in implementation. Broadly the challenges include engaging the public, collaboration, and partnerships, incorporating informatics, adopting a global approach, and assessing the impact of efforts. These challenges further change regionally owing to changes in the population and its response to the same drug.
To minimise the risk of or prevent ADRs, it is essential to keep sharing information and connect. Even though the ADRs may regionally differ, different pharmacovigilance units and healthcare agencies can collaborate to develop the best practices, guidelines, and safety profiles for the betterment of the overall population health and safety.
Future of Pharmacovigilance
Since the safety of a patient is paramount, pharmacovigilance systems are gaining increasing importance as organizations want accurate and thorough safety profiles on drugs. The governments and the regulatory authorities also want to ensure that every new drug/formulation that enters the market is well accepted and tolerated by the population at large.
Thus, today, the scope of drug safety surveillance is expansive and is becoming increasingly complex; the fact that the safety of a medicine is not dependent only on the pharmacological properties of the molecule but also to how a drug/formulation is used in actual practice by practitioners and to the maintenance of the product’s quality throughout the supply chain is well understood. While product quality concerns and prescription/usage patterns have always been important aspects of drug safety surveillance, their integration into pharmacovigilance systems has increased in the last decade.
As the Indian pharmaceutical industry continues to grow, the need for and the emphasis on pharmacovigilance is gaining momentum here too. And thus grows the need for professionals who can dexterously serve this role.
A Drug Safety Professional is one who fulfills the pharmacovigilance aspects of a drug. A pharmacist can fulfill this role efficiently, owing to the technical expertise and knowledge of drugs and their properties. Pharmacovigilance as a career option is undoubtedly a good choice for a pharmacist who is keen to actively work towards ensuring patient care safety and aims to prevent casualties due to adverse drug reactions. And is certainly a career that is currently in the boom phase.
References & Additional Reading:
- https://www.who.int
- https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5654220/
- https://globalpharmacovigilance.com
- https://www.who.int/medicines/publications/druginformation/issues
- https://link.springer.com/article/10.1007/s40264-013-0123-x
- https://www.ipc.gov.in/PvPI

