 Have you ever spared a thought on how a product that you consume gets the final green signal before it is introduced in the market? Each drug, cosmetic, food, beverage or supplement or for that matter any other item we use in our daily lives needs to be “approved” for consumption / utilisation by the consumers. Who does it and how is it done? Are there any regulations / guidelines prescribed by the government?
Have you ever spared a thought on how a product that you consume gets the final green signal before it is introduced in the market? Each drug, cosmetic, food, beverage or supplement or for that matter any other item we use in our daily lives needs to be “approved” for consumption / utilisation by the consumers. Who does it and how is it done? Are there any regulations / guidelines prescribed by the government?The healthcare industry is one of the few industries that witnesses innovations and new discoveries frequently. With research for newer therapeutic drugs and better delivery systems for patient care being the focus of this industry, there is continuous activity in almost all organisations. The huge base of R&D scientists, and professionals from the manufacturing and marketing functional areas fulfil this need for newer therapies. However, conceptualizing, manufacturing, packing and getting the end product to the market place is just the part that is generally the visible face of a large cycle discussed when a new product is launched.
Who helps the production and the marketing department of an organisation in making sure that the proposed new drug or formulation meets all the standards that are laid down for the consumption by the consumers? Is it safe enough, does it follow the required tolerability protocols and does it provide the efficacy that it claims to offer to the patients? Who gives the “licence” or the go ahead for it to reach the end consumers? These are some of the fundamental questions that any organisation looks into even before working on the production.
In every country it is the regulatory body or authority that controls the activities of every sector. And the pharma industry is no different. The countless stringent regulations and legislations that govern the entry of a drug into the market clearly demonstrate the controls that exist in this industry. From conception to execution, each step sees a great deal of involvement from the regulatory authorities. Each process requires approvals and permissions from the central or state authorities and a series of documents to be submitted to gain approval or license. We will give a brief overview of this process in India.
Regulatory Authority for Drugs & Pharmaceuticals in India
In India, the regulatory control over the pharmaceutical industry is exercised by the Central Drugs Standard Control Organisation (CDSCO) which lies under the Ministry of Health and Family Welfare. It is headed by the nodal agency called the Drug Controller General of India (DCGI).
“Under the Drug and Cosmetics Act, the regulation of manufacture, sale and distribution of drugs is primarily the concern of the State Authorities while the Central Authorities are responsible for approval of new drugs, clinical trials in the country, laying down the standards for drugs, control over the quality of imported drugs, coordination of the activities of State Drug Control Organisations and providing expert advice with a view to bring about the uniformity in the enforcement of the Drugs and Cosmetics Act.”
Further CDSCO along with state regulators, is jointly responsible for grant of licenses of certain specialized categories of critical drugs such as blood and blood products, I. V. Fluids, Vaccine and Sera.
The broad framework and functions of the CDSCO can be outlined as given below:

As the head of CDSCO, the DCGI exercises its policies and protocols through the Drugs and Cosmetics Act, 1940, and the Drugs Rules, 1945, which regulate the import, manufacture, licensing, testing, distribution, and sale of drugs in India and lay down general standards for all formulations.
The DCGI also coordinates the activities of the different State Drug Regulatory Authorities (SDRA). The DCGI is supported by two other boards/committees under the CDSCO - the Drugs Technical Advisory Board (DTAB) and the Drugs Consulting Committee (DCC). The DTAB advises the central government and the state governments on technical matters arising out of the administration of Drugs & Cosmetics Act and carry out functions assigned to it by this Act. The DCC advises the central and state governments as well as DTAB on any matter that tends to secure uniformity throughout India in the administration of this Act.
At this juncture, let’s look at the process of seeking approval for a new drug in India.
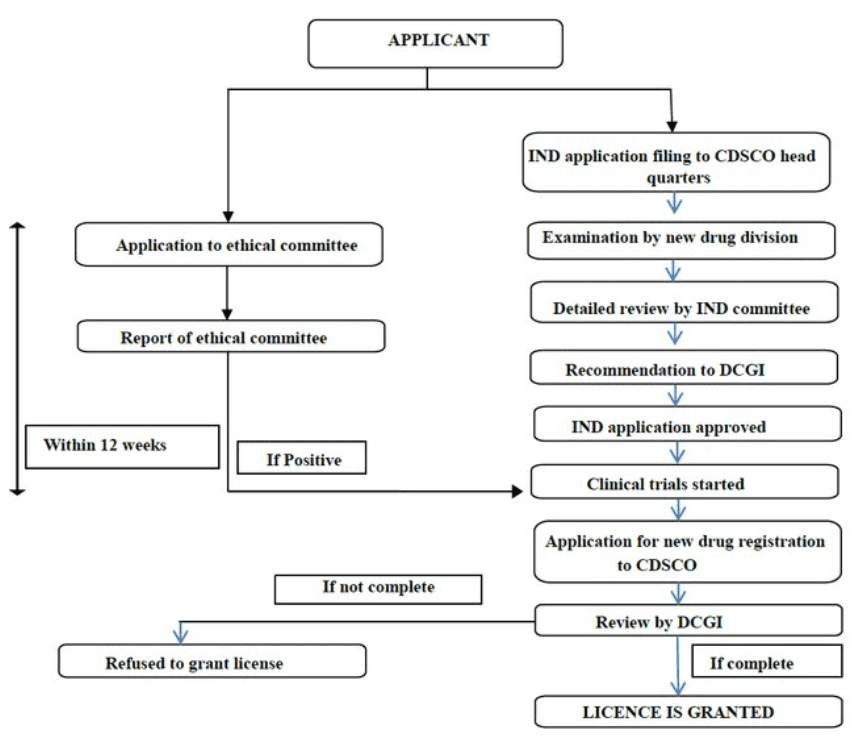
Source: https://www.researchgate.net/publication/327689192_
Regulatory controls at various stages of Drug Development and Marketing:
Any new drug or new formulation introduction by a company, undergoes various stages between its conceptualisation & research, production and finally marketing. The regulatory system in the country has put forth various Acts and Schedules that govern the functioning of each step in the process. Not just the licences and approvals, but also the framework and the guidelines that need to be complied with are laid down by the regulatory authorities. The major ones are as given below:
a) Pre- manufacturing:
This step involves all the processes involved even before the stage of production begins. After the R & D develops the drug, the drug has to go through several Clinical processes before a licence to manufacture is granted to an organisation.
The role of the regulatory body at this stage is to grant permission for production of the batches for clinical studies, approve, oversee and inspect clinical trials.
The Central Drugs Standard Control Organisation (CDSCO) and the Clinical Trials Registry-India (CTRI) are primarily responsible for overseeing these functions.
b) Manufacturing:
Every manufacturing set-up is required to comply with the Good Manufacturing Practices (GMP) guidelines laid down by the regulatory authorities. These guidelines clearly outline the requirements that need to be followed at different stages of the manufacture.
Schedule M to the Drugs & Cosmetics Act gives the directives to ensure that the manufacturing practices and requirements for manufacture of drugs and medical devices is proper and up-to-date and in harmony with the WHO and US-FDA protocols as well as the Indian FDA.
Schedule Y specifies guidelines for the conduct of clinical trials, import and manufacture of new drugs.
Every organization needs a license to be procured for the manufacture and marketing of a drug or formulation. This licence is granted by the State Drug Regulatory Authorities (SDRA).
c) Marketing, Sales and Distribution:
The marketing and sale, including export of pharmaceutical products requires various licences to be procured. This also is generally granted by the State Drug Regulatory Authorities (SDRA).
d) Pricing and Advertising :
Drug prices in India are regulated under the Drug Prices Control Order, 1995 (“DPCO”). It regulates the equitable distribution and increase in supply of a bulk drugs, and regulates the availability and fair price mechanism at which bulk drugs are sold.
The National Pharmaceutical Pricing Authority (NPPA) fixes or revises the prices of decontrolled bulk drugs and formulations at judicious intervals; periodically updates the list under price control through inclusion and exclusion of drugs in accordance with established guidelines; maintains data on production, exports and imports and market share of pharmaceutical firms; and enforces and monitors the availability of medicines in addition to imparting inputs to Parliament in issues pertaining to drug pricing.
The Drugs and Magic Remedies Act, 1954, prohibits the advertising of a drug in such a way that the advertisement contains any matter which directly or indirectly misrepresents the true character of the drug or makes a false claim or a claim which is false or misleading in any material particulars.
Regulatory Bodies outside of India
Every country has its own set of regulatory bodies which govern the manufacture and sale of drugs and formulations within their country. The US-FDA which operates in the United States; the Medicines and Healthcare Products Regulatory Agency (MHRA) regulates pharmaceuticals in the United Kingdom.
Apart from the regulatory bodies in respective countries, the FDA (Food and Drug Administration) has offices in various countries making it a regulatory body with an international presence.
Role of Regulatory Affairs Department in Organizations:
The regulatory affairs department has emerged as a very critical function in every organization today. This team provides the general strategy and direction to the project team to work within the regulatory guidelines. The regulatory affairs department functions as the critical intermediary between the organization and the regulatory authorities and ensures submission of the required paperwork and documents at various stages for procurement of the relevant approvals and licences in time. It also makes sure that the processes taking place are in accordance and in compliance with the protocols laid down by the regulatory bodies and plans the upcoming processes accordingly. It is the responsibility of the regulatory affairs department to be equipped with the latest developments and advances in the regulatory field, not just in their country, but across various regions in which the organization operates.
This function while is critical, also faces multitude challenges in our country owing to the very nature of the setup of this industry here. The drug pricing challenges, the highly fragmented nature with small companies and MNCs in the industry, ensuring manufacturing and quality standards as well as ethical practices in all areas are some of the major challenges faced by the central and state authorities. Despite the challenges, the Indian drug industry has made a great progress globally in the past few years.
The Pharmacist in Regulatory Function:
As a pharmacy graduate, one can have a promising career in the regulatory function of a pharmaceutical or allied organisation, helping and guiding the various departments in the organisation in the proper development of the products as well as their further entry into the market. One can begin the career straight after a Bachelor’s Degree or can look at entering this field after completing their post-graduation or doctorate degree in Pharmacy. In the last few years, because of the growing needs of this sector as well as the increasing scrutiny of the guidelines followed by the industry, there have been specialised courses designed specifically for students wanting to pursue a career in the regulatory field of the healthcare industry. These range from short certification courses to Master’s degree in niche areas.
In conclusion, the regulatory framework of the Indian healthcare industry is panacea for its existence and growth. The rapid progress that the pharma sector is currently witnessing calls for strict and vigilant regulations. The regulatory affairs field, like many others, is also currently going through a shift from the previous limited filing and submitting role to a more inclusive role which requires strategic planning, and the prediction & planning of the regulatory framework for a product from start to finish. As the processes and methods in the industry move towards a more sustainable and tech-oriented approach, it is the need-of-the-hour to have a regulatory affairs team with great strategic planning strength, excellent scientific and communication skills and a keen eye for everything that involves regulation. We thus enter an era which will clearly see a boom for pharmacists choosing this field as their career - a true BOSS in the pharma business.
References:
1. www.cdsco.gov.in
2. www.mhaonline.com
3. www.sciencedirect.com
4. www.cliniexperts.com
5. www.ipapharma.org
6. www.cuts-ccier.org

